Sunshine Factory, Co., Ltd. > Applications > ZrP for PolymerZrP for Polymer
A Brief Review on α-Zirconium Phosphate Intercalation Compounds and Nano-composites
1. Introduction
Due to the unique electronic and mechanical properties as intercalated by atoms, molecules, small organic groups and even polymers to change their properties significantly, layer structured materials are hot research topics.
Great progress has been achieved in the intercalation of the layer structured zirconium phosphate (ZrP) with organic groups and long chain polymers, and researches on the ZrP intercalation compounds are growing rapidly in recent years as they have been used widely in many fields, such as photochemistry, molecular and chiral recognition, biotechnologies and catalysis. The base structure of α-ZrP does not change appreciably under mild conditions (ion exchange, intercalation, topotactic exchange, infinite swelling, etc.). α-ZrP is insoluble and very stable toward thermal treatment and wild chemical environment, such as strong acids/bases and redox agents .
Due to the unique electronic and mechanical properties as intercalated by atoms, molecules, small organic groups and even polymers to change their properties significantly, layer structured materials are hot research topics.
Great progress has been achieved in the intercalation of the layer structured zirconium phosphate (ZrP) with organic groups and long chain polymers, and researches on the ZrP intercalation compounds are growing rapidly in recent years as they have been used widely in many fields, such as photochemistry, molecular and chiral recognition, biotechnologies and catalysis. The base structure of α-ZrP does not change appreciably under mild conditions (ion exchange, intercalation, topotactic exchange, infinite swelling, etc.). α-ZrP is insoluble and very stable toward thermal treatment and wild chemical environment, such as strong acids/bases and redox agents .
A brief review on the α-ZrP intercalation compounds
Figure 1 is a schematic representation of the arrangement of three layers of α-ZrP. The crystal structure is monoclinic in space group of P21/n with a=0.9060 nm, b=0.5297 nm,c=1.5414 nm and bβ=101.71°. In each layer, a plane of Zr atoms is sandwiched between two layers of tetrahedral phosphate groups and Zr atoms locate alternatively above and below the mean plane. Each Zr atom is octahedrally coordinated by six oxygen atoms from six different phosphate groups. Thus, three oxygen atoms of each phosphate are bonded to three different zirconium atoms arranged at the apices of a near equilateral triangle.
The fourth oxygen atom of phosphate protrudes into the interlayer space as shown in Figure 1.
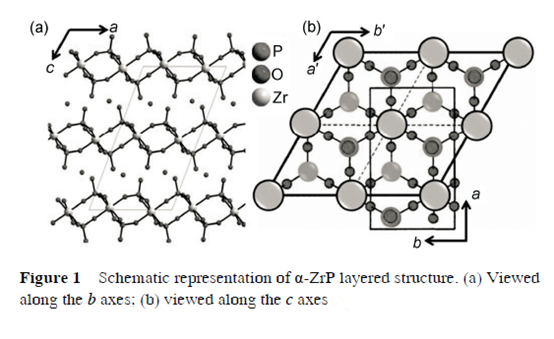
2. α-ZrP intercalation compounds
The unique layered structure and flexibility of α-ZrP permit ions, small organic groups and even long-chain polymers to intercalate into the interfacial region. For small ions, intercalation is mainly via ion diffusion and exchanges with the H atom of the -OH group. For example, a limited number of cations (Li+, Na+, Ag+, Cu+ and Ca2+) can exchange with the protons of α-ZrP at a high rate in acid solutions. Large monovalent and divalent cations (such as NH4+, Rb2+, and Ba2+) or highly hydrated divalent and trivalent cations (such as Mg2+,Cu2+, Cr3+ and Rh3+) are also able to replace the protons of α-ZrP, but the rates are very slow.
Usually, precursors with a large interlayer separation , such as α-Zr(HPO4)(NaPO4)·5H2O and intercalation compounds with ethanol or alkylamine, can be employed to facilitate the exchange of large size or highly hydrated cations. Experimental results show that even the small ion intercalated α-ZrP can improve their performance significantly. For example, both Cu and Fe intercalated α-ZrP are very efficient catalysts. Small organic groups -R usually substitute the –OH group in the phosphonates of α-ZrP with its layered inorganic network essentially unaltered. A more detailed description about organic derivatives of aα-ZrP with -R pendant groups is referred to refs. More recently, Amicangelo synthesized some organic derivatives of a-ZrP, which have general formula of Zr(O3PR)1(O3PR′)1 with R and R′ being -C10H7, -C14H9, -OC4H9, or -OC2H5
(Figure 2).
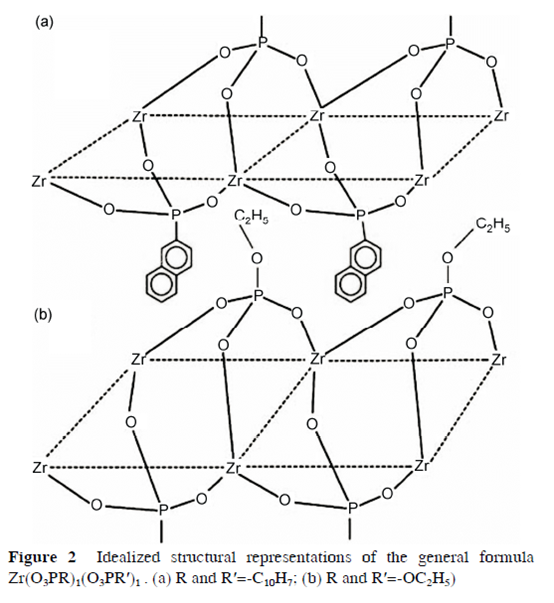
Accordingly, several experimental methods have been developed to synthesize polymer/α-ZrP inorganic nano-composites, including exfoliation-adsorption, in-situ intercalative polyme- rization, melt intercalation and template synthesis.
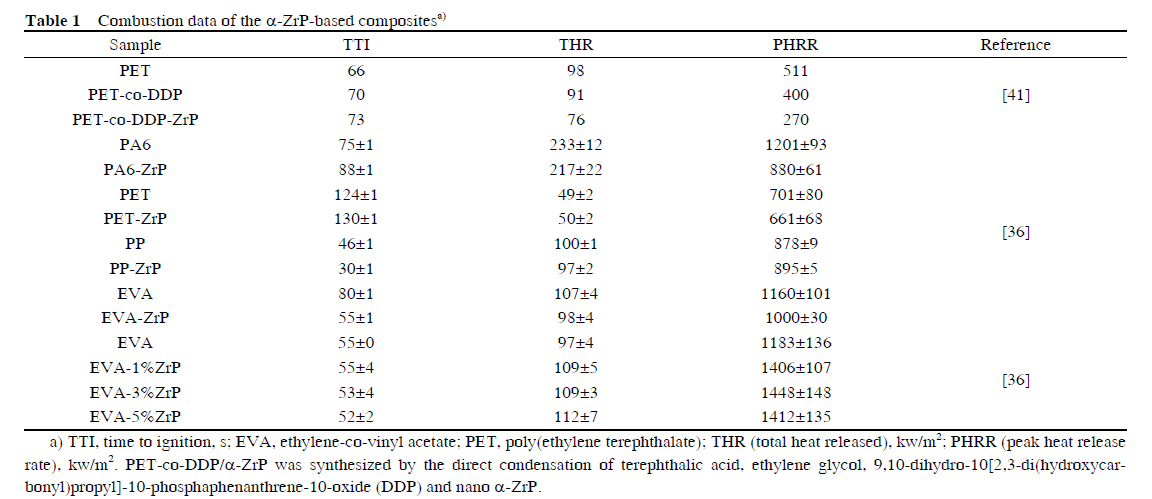
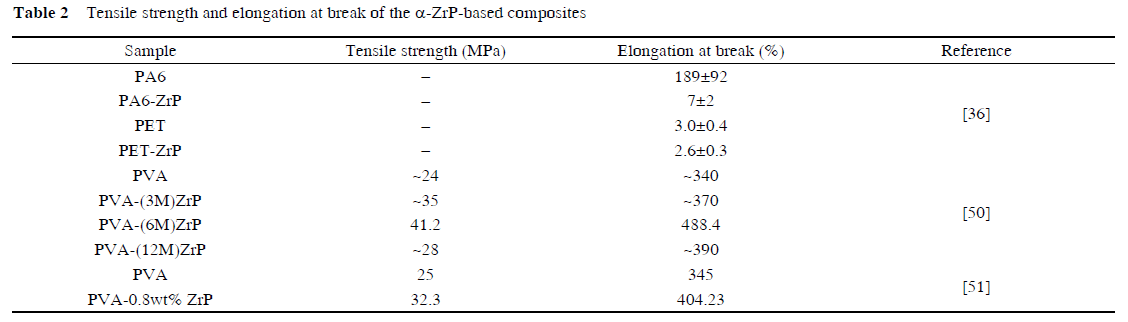
There have been several experimental investigations on polymer/ α-ZrP nano-composites including poly (ethylene terephthalate)(PET)/α-ZrP, epoxy/α-ZrP, polyacrylamide(PAM)/α-ZrP, poly(vinyl alcohol)(PVA)/α-ZrP, and starch/α-ZrP. These nano-composites will be reviewed in detail in the following paragraphs and the combustion and mechanical properties of some nano-composites are listed in Tables 1 and 2.

Follow WeChat


