Sunshine Factory, Co., Ltd. > Applications > ZrP for PolymerZrP for Polymer
Thermal Stability of Optically Transparent Alpha-Zirconium Phosphate/Poly(Methyl Methacrylate) Nanocomposites with High Particle Loading
Abstract
Nanocomposites comprised of alpha-zirconium phosphate (ZrP) nanosheets in poly (methyl methacrylate) (PMMA) were prepared with a wide range of nanoparticle loadings (0, 5, 10, and 30 wt.% ZrP in PMMA). The ZrP nanocomposites were characterized using UV-visible spectroscopy (UV-vis), x-ray diffraction (XRD), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).
Nanocomposites were well dispersed and optically transparent as shown by XRD and UV-vis. However in the UV region, transparent ZrP nanocomposites possessed excellent UV scattering properties, significantly reducing the transmittance of UV-light, while remaining transparent to the visual spectrum. Thermal stability studies using TGA and DTG showed the peak mass loss rate (PMLR) was reduced by 10% and simultaneously shifted to higher temperatures by 41 °C. Since the nanocomposites in this work cover such a large range of ZrP loadings, large amounts of high temperature residuals were encountered after TGA studies, indicating that the high loading ZrP nanocomposites are largely noncombustible. In addition, DSC studies showed that ZrP content does affect the glass transition temperature, but not enough to limit the application in which ZrP nanocomposites could be used.
These results point to ZrP nanocomposites being useful as polymer replacements, behaving like polymers until the event of a fire, in which case they are largely noncombustible.
Preparation of ZrP/PMMA Nanocomposites
Nanocomposites were produced in the range of 0 wt.% to 30 wt.% ZrP inPMMA. This was accomplished by first mixing a solution of PMMA (15 wt.%PMMA in DMF) with an appropriate amount of ZrP suspension in DMF for 15 min. The solution was sonicated for 15 minutes before being cast onto tin foil-lined glass dishes. Samples were dried at 100 °C for 24 h, after which the foil was removed from the dried sample to produce a single free-standing ZrP/ PMMA nanocomposite film.
Results and Discussion
1. Nanocomposite Morphology
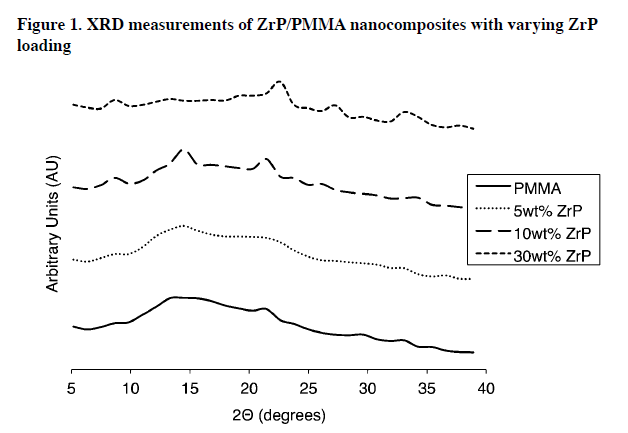
XRD was used to study the internal morphology of the prepared nanocomposites.
As seen in Figure 1, pure PMMA is amorphous with verybroad peaks around 14 and 21°, corresponding to polymer chain-tochain packing and intermolecular distances in PMMA, respectively. At 5 wt.% ZrP in PMMA, a peak appears at 8.30°, but since the peak associated with the layer-to-layer distance of agglomerated ZrP (7.6 Å, 11.6°) is not present, this peak indicates the presence of intercalated ZrP.
As the loading of ZrP in PMMA is increased further, the intercalation peak also increases indicating the concentration of intercalated ZrP increases with ZrP loading. Small peaks at 19.5°,22.2°, 27.4°, and 33.9° approximately correspond to peaks found in the literature for the intercalated ZrP gel: 20.1°, 22.0°, 29.1°, and 33.5°, respectively.
 2. Optical Properties
2. Optical Properties
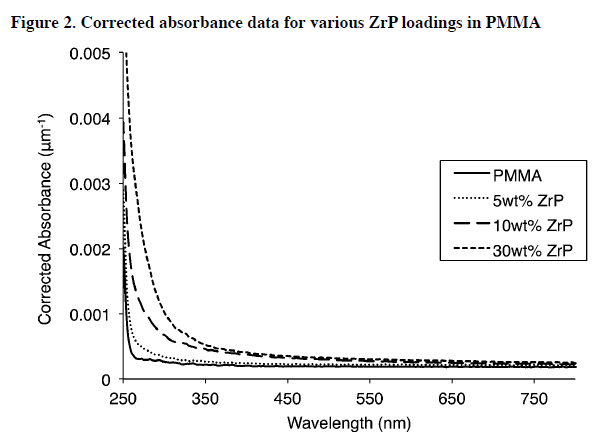
The effects of ZrP concentration on PMMA transparency and UV absorption was tested using UV-vis spectroscopy.
To take into account the varying thickness of the nanocomposite samples, the corrected absorbance, (absorbance divided by the sample film thickness) is reported for the wavelength range of 250 nm to 700 nm and shown in Figure 2
It is apparent that even up to 30 wt.% ZrP, samples are remarkably transparent throughout the visible spectrum. Unlike many other PMMA nanocomposites, ZrP/PMMA nanocomposites are optically transparent even at the high ZrP loadings seen in this study.
Figure 2 also shows that increasing ZrP content also increases UV scattering and absorption, similarly to other metal oxides. This gives ZrP/PMMA nanocomposites the unique ability to remain optically transparent while rejecting significant amounts of UV light.
 3. Thermal Stability Studies
3. Thermal Stability Studies
The thermal stability of the ZrP/PMMA nanocomposites was analyzed using TGA (Figure 3) and the corresponding DTG (Figure 4). The DTG data in Figure 4 shows there is one main degradation reaction for each sample.
Since these studies were done it air, it is expected that PMMA will have a single, broad degradation reaction, as shown by Kashiwagi. It is important to note that, although the general shapes of TGA curves in Figure 3 are similar, the corresponding DTG plots show a dramatic shift in the peak mass loss rate (PMLR) to higher temperatures (41 °C higher), while simultaneously reducing the peak by 10%.
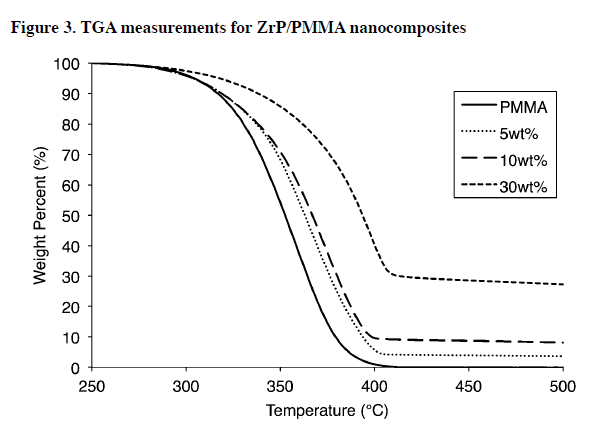
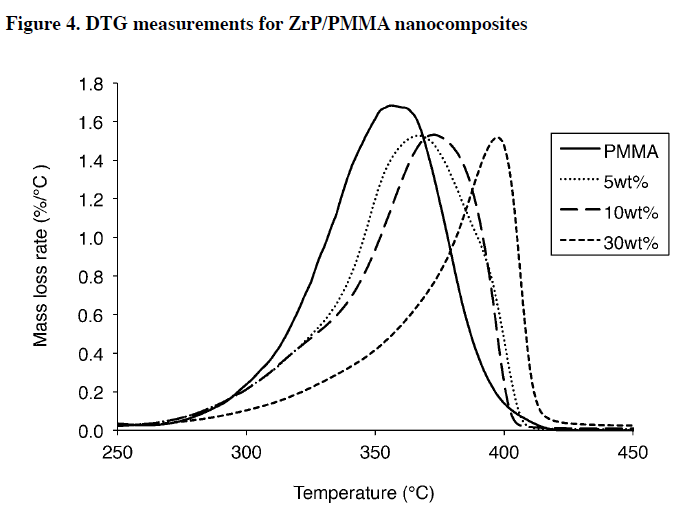 Table 1 summarizes the onset of degradation (temperature at 5% mass loss), the temperature at 25% mass loss, the temperature at 50% mass loss, and the temperature at the PMLR.
Table 1 summarizes the onset of degradation (temperature at 5% mass loss), the temperature at 25% mass loss, the temperature at 50% mass loss, and the temperature at the PMLR.
Table 1 shows that the onset of degradation is affected slightly by the presence of ZrP, shifting it towards higher temperatures, for the 30 wt.% ZrP nanocomposite. The temperature for 25% mass loss and 50% mass loss are shifted dramatically by a maximum of 34 °C and 41 °C, respectively.
Finally, the temperature at which the peak mass loss rate occurs is shifted upwards by 41 °C, showing that the ZrP nanocomposites are significantly more thermally stable when compared to neat PMMA. Improvements to thermal stability are significant, even in comparison to other commonly used nanofillers such as montmorillonite, aluminum oxide, and boehmite particle (AlOOH). It is possible the thermal stability effects are caused by a trapping mechanism or decreased polymer mobility due to nanofillers, similar to other nanocomposites
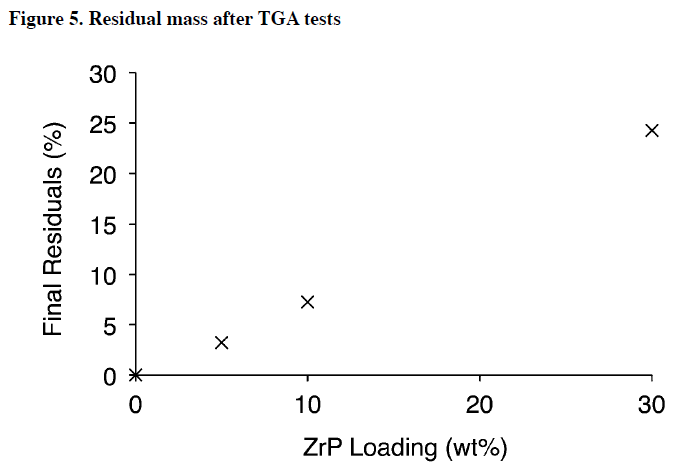
The presence of high temperature residuals in Figure 3 suggests there is a dramatic enhancement in the retention of mass. The final mass of samples after TGA tests is summarized in Figure 5 and shows ZrP nanocomposites have enhanced residuals, due in part to their thermally stable inorganic ZrP content. This is one of the benefits of high loadings ZrP nanocomposites.
Under normal conditions, ZrP nanocomposites look and act similar to a polymer, but in the event of a fire, the material is largely noncombustible. Compared to numerous other nanofillers in literature, the materials in this study provide superior high temperature residuals
 4. Glass Transition Temperature Effects
4. Glass Transition Temperature Effects
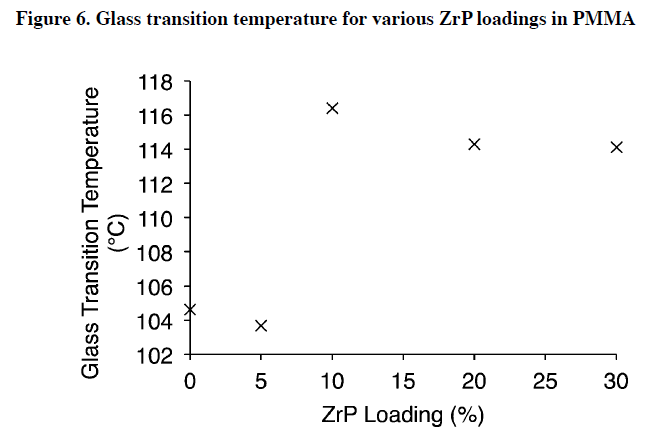
Results from the DSC tests were used to understand how ZrP content affected glass transition temperatures, and are shown in Figure 6.
At loadings below 10 wt.%, addition of ZrP increases the glass transition temperature. Weight loadings greater than 10 wt.% ZrP begin to decrease the glass transition temperature. Literature suggests that exfoliatedceramic plates in PMMA decrease the mobility of polymer chains leading to an increase in the glass transition temperature.
Figure 6 shows a similar for the same range of nanofiller loading (below 5 wt.%). However,above 10 wt.%, the data show a decrease in glass transition temperature which is most likely due to nanoparticle agglomeration. Large nanoparticle agglomerates act as a single particle, but contain many individual nanosheets, effectively reducing the number of individual particles seen by the polymer matrix. This effect decreases the glass transition temperature of the bulk material at higher loadings.
 Conclusions
Conclusions
Nanocomposites of ZrP in PMMA with a wide range of nanoparticle loadings (0-30 wt.%) were produced using a mild synthesis process at room temperature followed by solution casting. The nanocomposites were homogeneous and showed excellent optical transparency, while simultaneously scattering significant amounts of UV light.
Thermal stability studies using TGA showed that all materials degrade in a single broad reaction when exposed to air. However, the peak mass loss rate (PMLR) for ZrP nanocomposites was reduced by 10% and shifted up 41 °C when compared to neat PMMA, showing these nanocomposites possess enhanced thermal stability. In addition, the high loadings of the ZrP nanocomposites in this study allow for increased residuals at high temperature. This means that ZrP nanocomposites can be used in traditional polymeric applications, but in the event of a fire, are largely noncombustible.
Lastly, the DSC tests showed that the glass transition temperature does change with respect to ZrP loading, but the maximum difference observed was only 12 °C, indicating that the applications of ZrP nanocomposites should not be hindered by their glass transition.
Nanocomposites comprised of alpha-zirconium phosphate (ZrP) nanosheets in poly (methyl methacrylate) (PMMA) were prepared with a wide range of nanoparticle loadings (0, 5, 10, and 30 wt.% ZrP in PMMA). The ZrP nanocomposites were characterized using UV-visible spectroscopy (UV-vis), x-ray diffraction (XRD), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC).
Nanocomposites were well dispersed and optically transparent as shown by XRD and UV-vis. However in the UV region, transparent ZrP nanocomposites possessed excellent UV scattering properties, significantly reducing the transmittance of UV-light, while remaining transparent to the visual spectrum. Thermal stability studies using TGA and DTG showed the peak mass loss rate (PMLR) was reduced by 10% and simultaneously shifted to higher temperatures by 41 °C. Since the nanocomposites in this work cover such a large range of ZrP loadings, large amounts of high temperature residuals were encountered after TGA studies, indicating that the high loading ZrP nanocomposites are largely noncombustible. In addition, DSC studies showed that ZrP content does affect the glass transition temperature, but not enough to limit the application in which ZrP nanocomposites could be used.
These results point to ZrP nanocomposites being useful as polymer replacements, behaving like polymers until the event of a fire, in which case they are largely noncombustible.
Preparation of ZrP/PMMA Nanocomposites
Nanocomposites were produced in the range of 0 wt.% to 30 wt.% ZrP inPMMA. This was accomplished by first mixing a solution of PMMA (15 wt.%PMMA in DMF) with an appropriate amount of ZrP suspension in DMF for 15 min. The solution was sonicated for 15 minutes before being cast onto tin foil-lined glass dishes. Samples were dried at 100 °C for 24 h, after which the foil was removed from the dried sample to produce a single free-standing ZrP/ PMMA nanocomposite film.
Results and Discussion
1. Nanocomposite Morphology
XRD was used to study the internal morphology of the prepared nanocomposites.
As seen in Figure 1, pure PMMA is amorphous with verybroad peaks around 14 and 21°, corresponding to polymer chain-tochain packing and intermolecular distances in PMMA, respectively. At 5 wt.% ZrP in PMMA, a peak appears at 8.30°, but since the peak associated with the layer-to-layer distance of agglomerated ZrP (7.6 Å, 11.6°) is not present, this peak indicates the presence of intercalated ZrP.
As the loading of ZrP in PMMA is increased further, the intercalation peak also increases indicating the concentration of intercalated ZrP increases with ZrP loading. Small peaks at 19.5°,22.2°, 27.4°, and 33.9° approximately correspond to peaks found in the literature for the intercalated ZrP gel: 20.1°, 22.0°, 29.1°, and 33.5°, respectively.

The effects of ZrP concentration on PMMA transparency and UV absorption was tested using UV-vis spectroscopy.
To take into account the varying thickness of the nanocomposite samples, the corrected absorbance, (absorbance divided by the sample film thickness) is reported for the wavelength range of 250 nm to 700 nm and shown in Figure 2
It is apparent that even up to 30 wt.% ZrP, samples are remarkably transparent throughout the visible spectrum. Unlike many other PMMA nanocomposites, ZrP/PMMA nanocomposites are optically transparent even at the high ZrP loadings seen in this study.
Figure 2 also shows that increasing ZrP content also increases UV scattering and absorption, similarly to other metal oxides. This gives ZrP/PMMA nanocomposites the unique ability to remain optically transparent while rejecting significant amounts of UV light.

The thermal stability of the ZrP/PMMA nanocomposites was analyzed using TGA (Figure 3) and the corresponding DTG (Figure 4). The DTG data in Figure 4 shows there is one main degradation reaction for each sample.
Since these studies were done it air, it is expected that PMMA will have a single, broad degradation reaction, as shown by Kashiwagi. It is important to note that, although the general shapes of TGA curves in Figure 3 are similar, the corresponding DTG plots show a dramatic shift in the peak mass loss rate (PMLR) to higher temperatures (41 °C higher), while simultaneously reducing the peak by 10%.


Table 1 shows that the onset of degradation is affected slightly by the presence of ZrP, shifting it towards higher temperatures, for the 30 wt.% ZrP nanocomposite. The temperature for 25% mass loss and 50% mass loss are shifted dramatically by a maximum of 34 °C and 41 °C, respectively.
Finally, the temperature at which the peak mass loss rate occurs is shifted upwards by 41 °C, showing that the ZrP nanocomposites are significantly more thermally stable when compared to neat PMMA. Improvements to thermal stability are significant, even in comparison to other commonly used nanofillers such as montmorillonite, aluminum oxide, and boehmite particle (AlOOH). It is possible the thermal stability effects are caused by a trapping mechanism or decreased polymer mobility due to nanofillers, similar to other nanocomposites

Under normal conditions, ZrP nanocomposites look and act similar to a polymer, but in the event of a fire, the material is largely noncombustible. Compared to numerous other nanofillers in literature, the materials in this study provide superior high temperature residuals

Results from the DSC tests were used to understand how ZrP content affected glass transition temperatures, and are shown in Figure 6.
At loadings below 10 wt.%, addition of ZrP increases the glass transition temperature. Weight loadings greater than 10 wt.% ZrP begin to decrease the glass transition temperature. Literature suggests that exfoliatedceramic plates in PMMA decrease the mobility of polymer chains leading to an increase in the glass transition temperature.
Figure 6 shows a similar for the same range of nanofiller loading (below 5 wt.%). However,above 10 wt.%, the data show a decrease in glass transition temperature which is most likely due to nanoparticle agglomeration. Large nanoparticle agglomerates act as a single particle, but contain many individual nanosheets, effectively reducing the number of individual particles seen by the polymer matrix. This effect decreases the glass transition temperature of the bulk material at higher loadings.

Nanocomposites of ZrP in PMMA with a wide range of nanoparticle loadings (0-30 wt.%) were produced using a mild synthesis process at room temperature followed by solution casting. The nanocomposites were homogeneous and showed excellent optical transparency, while simultaneously scattering significant amounts of UV light.
Thermal stability studies using TGA showed that all materials degrade in a single broad reaction when exposed to air. However, the peak mass loss rate (PMLR) for ZrP nanocomposites was reduced by 10% and shifted up 41 °C when compared to neat PMMA, showing these nanocomposites possess enhanced thermal stability. In addition, the high loadings of the ZrP nanocomposites in this study allow for increased residuals at high temperature. This means that ZrP nanocomposites can be used in traditional polymeric applications, but in the event of a fire, are largely noncombustible.
Lastly, the DSC tests showed that the glass transition temperature does change with respect to ZrP loading, but the maximum difference observed was only 12 °C, indicating that the applications of ZrP nanocomposites should not be hindered by their glass transition.

Follow WeChat


