Sunshine Factory, Co., Ltd. > Applications > ZrP for CoatingZrP for Coating
Advanced Anticorrosion Coatings Prepared from Polybenzoxazine/α-zirconium Phosphate Nanocomposites
Abstract
In this study, we presented the first successful application of polybenzoxazine/α-Zirconium Phosphate (PBa/α-ZrP) nanocomposites for corrosion protection. α-ZrP nanoplatelets were exfoliated by a polyetheramine surfactant (Jeffamine M1000), and the α-ZrP dispersed well in the PBa matrix with no substantial agglomeration. PBa/α-ZrP composite coatings were also prepared on the carbon steel substrate, and their anticorrosive properties were investigated in 3.5 wt% NaCl solution via electrochemical impedance spectroscopy and polarization curves.
The results revealed that well-dispersed α-ZrP nanoplatelets in the PBa matrix effectively enhanced the corrosion protection ability of the coatings, attributing to the extended diffusion path of the corrosive agents in the coating to reach to the protected metal surface. The samples with 5 wt% α-ZrP nanoplatelets showed a corrosion rate of 0.108 mm per year for carbon steel after 8-day immersion, and the corrosion rate was less than half of that of the neat pristine PBa sample. These results of this study reveal that the incorporation of α-ZrP in the PBa matrix efficiently improved the corrosion protection of the PBa composite.
Preparation of PBa/α-ZrP nanocomposite coatings
In this study, we presented the first successful application of polybenzoxazine/α-Zirconium Phosphate (PBa/α-ZrP) nanocomposites for corrosion protection. α-ZrP nanoplatelets were exfoliated by a polyetheramine surfactant (Jeffamine M1000), and the α-ZrP dispersed well in the PBa matrix with no substantial agglomeration. PBa/α-ZrP composite coatings were also prepared on the carbon steel substrate, and their anticorrosive properties were investigated in 3.5 wt% NaCl solution via electrochemical impedance spectroscopy and polarization curves.
The results revealed that well-dispersed α-ZrP nanoplatelets in the PBa matrix effectively enhanced the corrosion protection ability of the coatings, attributing to the extended diffusion path of the corrosive agents in the coating to reach to the protected metal surface. The samples with 5 wt% α-ZrP nanoplatelets showed a corrosion rate of 0.108 mm per year for carbon steel after 8-day immersion, and the corrosion rate was less than half of that of the neat pristine PBa sample. These results of this study reveal that the incorporation of α-ZrP in the PBa matrix efficiently improved the corrosion protection of the PBa composite.
Preparation of PBa/α-ZrP nanocomposite coatings
The Q235 carbon steel samples (10*10*1mm3) were first cleaned and degreased with acetone.The viscous dichloromethane solution containing Ba/α-ZrP with a concentration of 0.5 mg/mL-1 was then drop cast onto the substrate, resulting in the formation of a solid film after the evaporation of the volatile solvent at 50 °C.The PBa/α-ZrP coated electrode was obtained subsequently by heating the PBa/α-ZrP-covered working electrode at 180 °C for 2 h and 200 °C for 2h. AB glue was used for sample sealing.
RESULTS AND DISCUSSION
1. Characterization of PBa/α-ZrP composite coatings
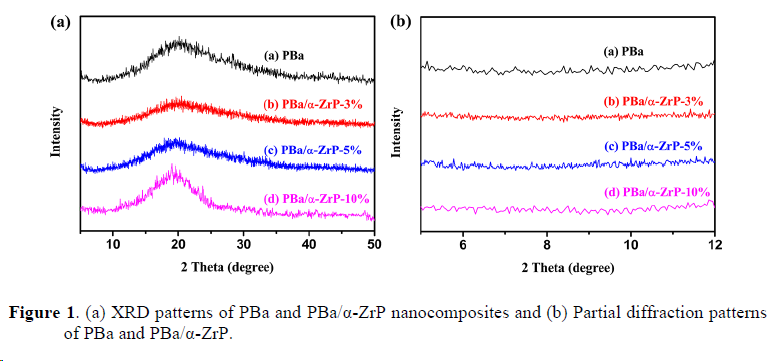

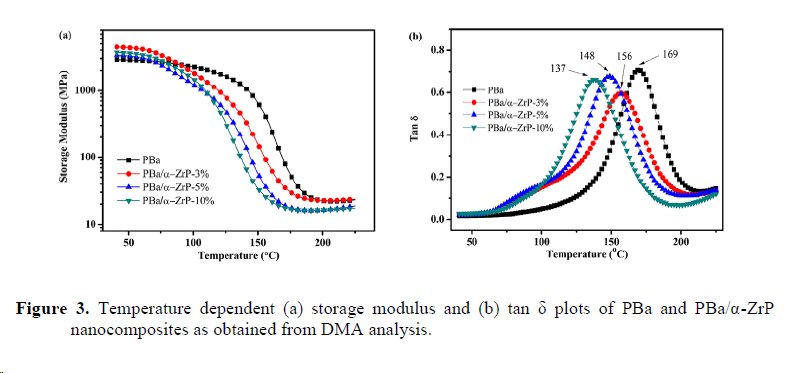
2. Thermal dynamic mechanical characterization
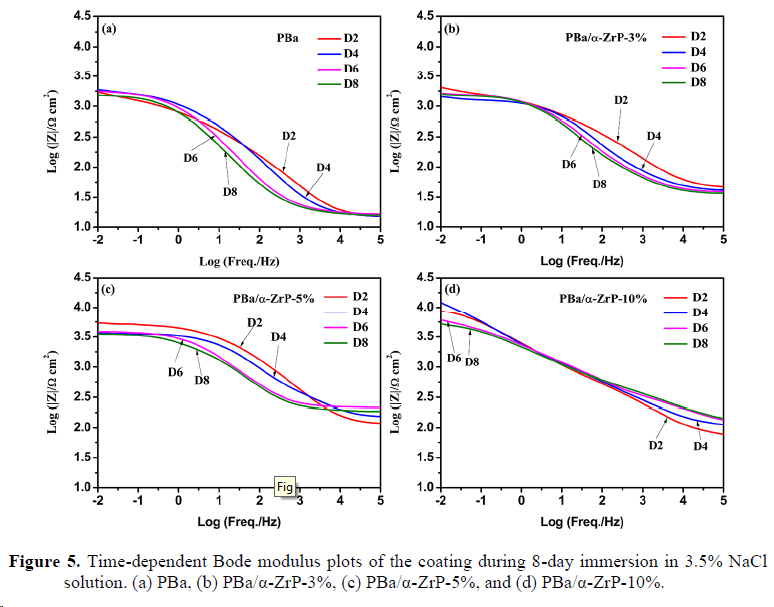
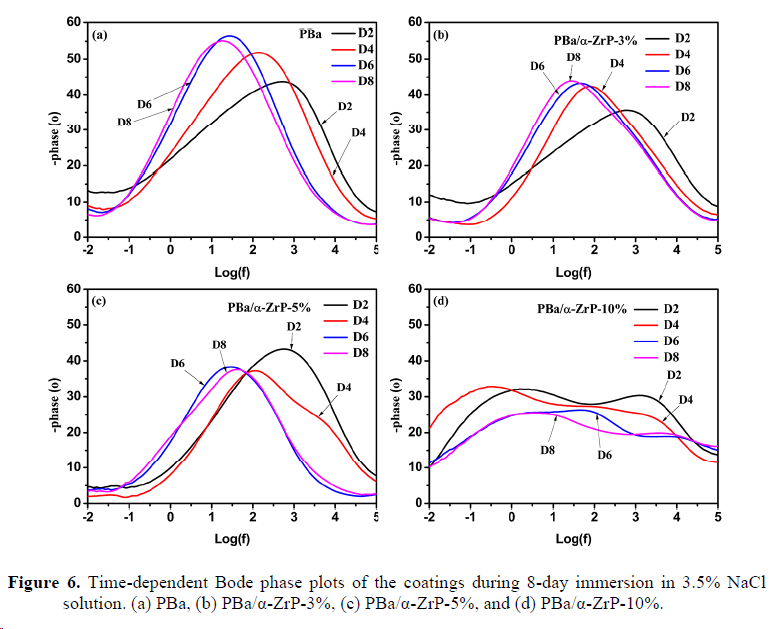
3. Anticorrosive performance of the electrode coated with different coatings

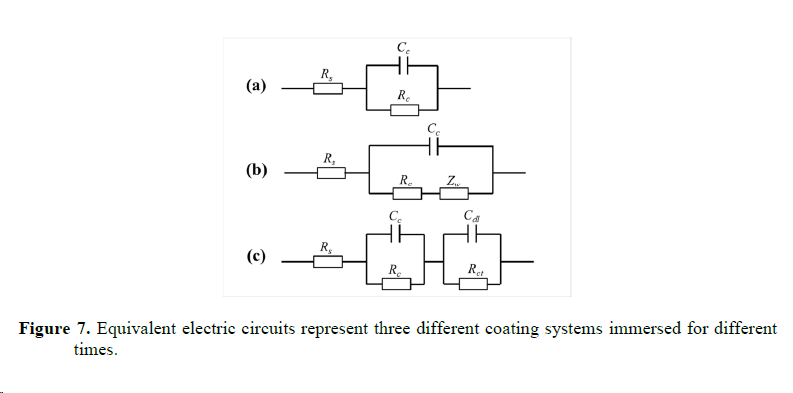
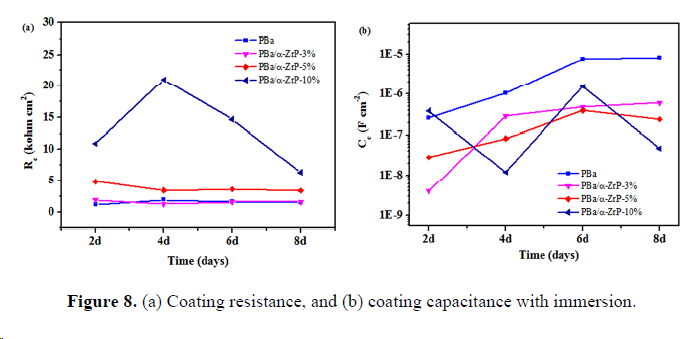
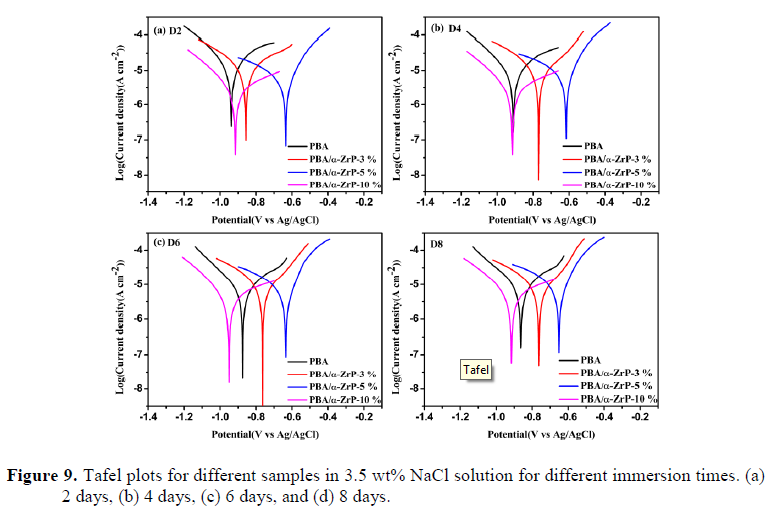
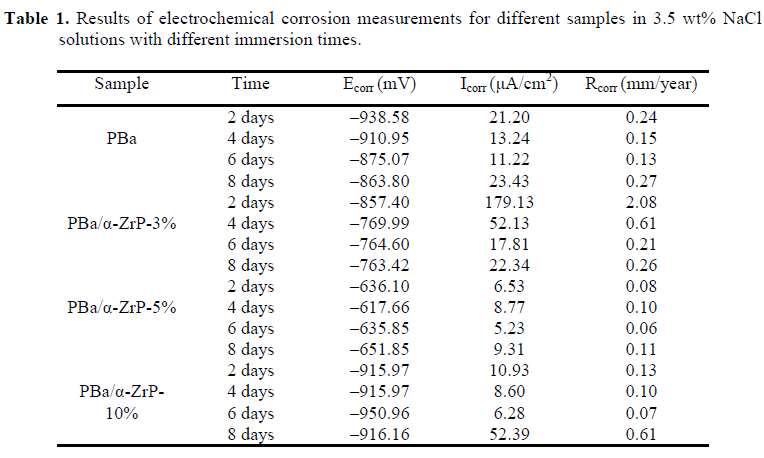
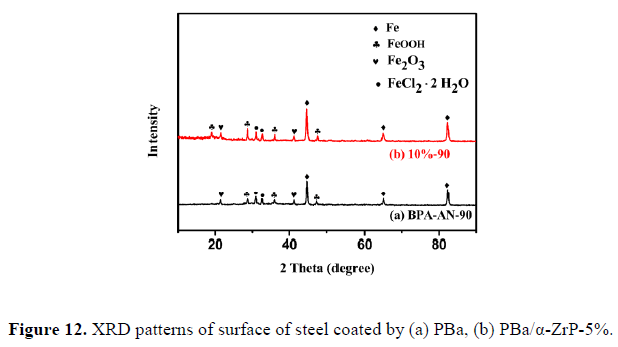
4. Characterization of corrosion products


CONCLUSION
We successfully prepared multifunctional PBa/α-ZrP nanocomposite coatings, ideally suitable for field applications, where high corrosion resistance is required. The exfoliated α-ZrP nanoplates dispersed well in the PBa matrix, and no substantial agglomeration occurred as evidenced by the XRD, SEM, EDX, and TEM analyses, attributing to the homogeneous nanoscopic dispersion of the exfoliated α-ZrP nanoplates and the extended diffusion path of corrosive agents to reach the protected metal surface. The incorporation of α-ZrP significantly enhanced the corrosion protection performance of PBa coatings as proven by the potentiodynamic polarization curves and EIS analysis. Further, PBa/α-ZrP-5% exhibited upper impedance modulus, more positive Ecorr, lower Icorr, and declined Rcorr during the 8-day immersion, while the pristine PBa coating did not show the same result under the same test conditions. The morphologies of the corrosion products were investigated by SEM, and the elemental components were measured by XRD and EDX. Overall, the incorporation of α-ZrP in the PBa matrix can enhance the anticorrosive properties of PBa composites.
RESULTS AND DISCUSSION
1. Characterization of PBa/α-ZrP composite coatings


2. Thermal dynamic mechanical characterization

3. Anticorrosive performance of the electrode coated with different coatings







4. Characterization of corrosion products



CONCLUSION
We successfully prepared multifunctional PBa/α-ZrP nanocomposite coatings, ideally suitable for field applications, where high corrosion resistance is required. The exfoliated α-ZrP nanoplates dispersed well in the PBa matrix, and no substantial agglomeration occurred as evidenced by the XRD, SEM, EDX, and TEM analyses, attributing to the homogeneous nanoscopic dispersion of the exfoliated α-ZrP nanoplates and the extended diffusion path of corrosive agents to reach the protected metal surface. The incorporation of α-ZrP significantly enhanced the corrosion protection performance of PBa coatings as proven by the potentiodynamic polarization curves and EIS analysis. Further, PBa/α-ZrP-5% exhibited upper impedance modulus, more positive Ecorr, lower Icorr, and declined Rcorr during the 8-day immersion, while the pristine PBa coating did not show the same result under the same test conditions. The morphologies of the corrosion products were investigated by SEM, and the elemental components were measured by XRD and EDX. Overall, the incorporation of α-ZrP in the PBa matrix can enhance the anticorrosive properties of PBa composites.

Follow WeChat


