Sunshine Factory, Co., Ltd. > Applications > ZrP for Water TreatmentZrP for Water Treatment
A novel organic-inorganic hybrid film consisting of polypyrrole/α-zirconium phosphate(PPy/α-ZrP) was prepared in an aqueous phase by cyclic voltammetry method. It was proved by FT-IR, XRD and XPS characterizations that the electroactive PPy/α-ZrP hybrid film was synthesized successfully.
The PPy/α-ZrP film electrode prepared on a porous three-dimensional carbon felt(PTCF) was used for removal of a low concentration of Pb(Ⅱ) ions from an aqueous solution by electrochemically switched ion exchange(ESIX).
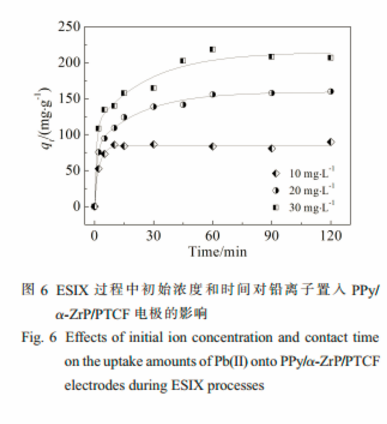
A quick uptake and release rate of Pb(Ⅱ) onto the film electrode was obtained by adjusting redox state of the hybrid film.
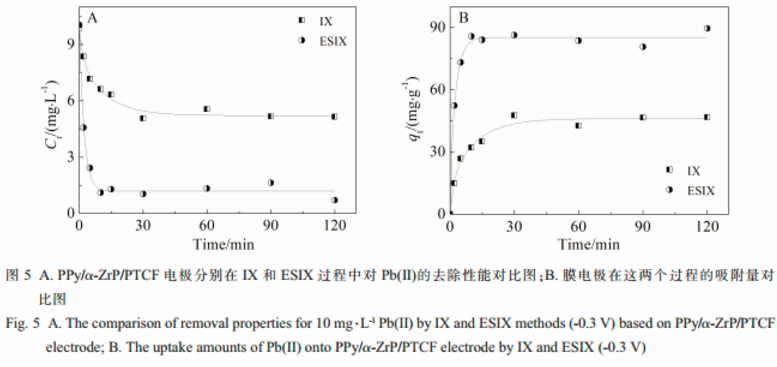
The removal efficiency and adsorption capacity of the PPy/α-ZrP/PTCF electrode for Pb(Ⅱ) were about 1.8 and 2 times higher than those of traditional ion exchange(IX), respectively. Thus, the film electrode showed higher removal efficiency and adsorption capacity for Pb(Ⅱ) by ESIX.
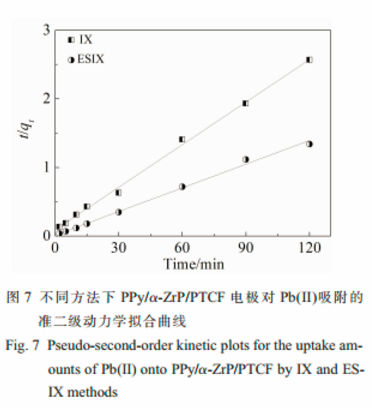
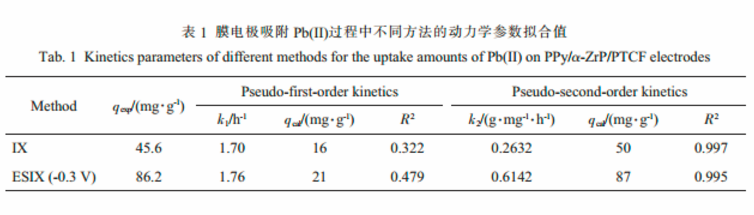
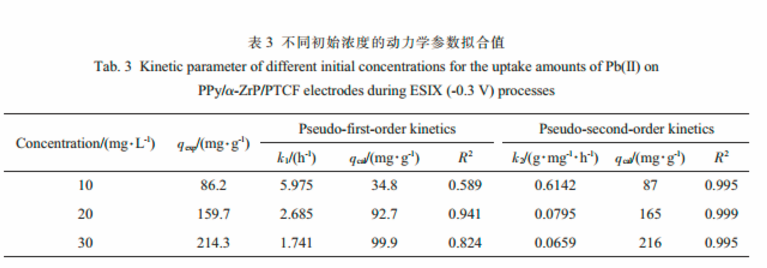
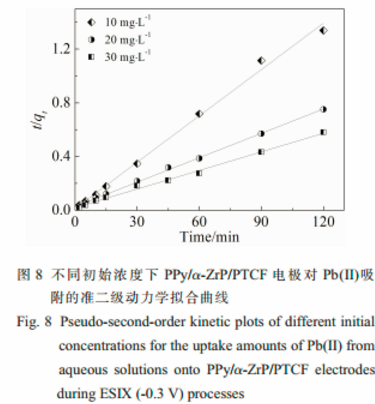
The adsorption kinetics of Pb(Ⅱ) could be described properly by the pseudo-second-order kinetic mode.
A greater pseudo-second-order rate constant of 0.6142 g·mg-1·h-1was achieved by ESIX, which was higher than that of 0.2632 g·mg-1·h-1by IX.
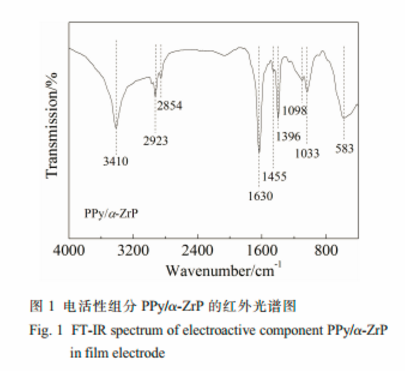
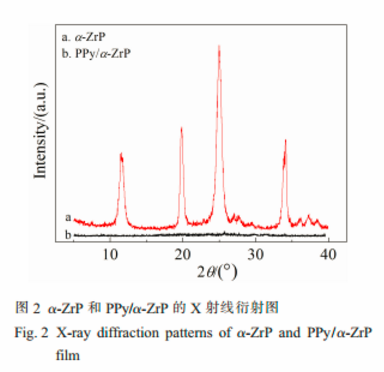
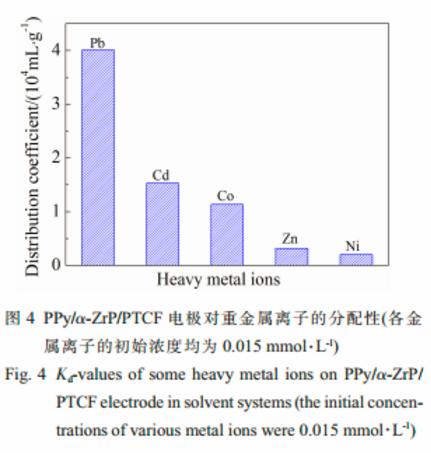

The PPy/α-ZrP film electrode prepared on a porous three-dimensional carbon felt(PTCF) was used for removal of a low concentration of Pb(Ⅱ) ions from an aqueous solution by electrochemically switched ion exchange(ESIX).
A quick uptake and release rate of Pb(Ⅱ) onto the film electrode was obtained by adjusting redox state of the hybrid film.
The removal efficiency and adsorption capacity of the PPy/α-ZrP/PTCF electrode for Pb(Ⅱ) were about 1.8 and 2 times higher than those of traditional ion exchange(IX), respectively. Thus, the film electrode showed higher removal efficiency and adsorption capacity for Pb(Ⅱ) by ESIX.
The adsorption kinetics of Pb(Ⅱ) could be described properly by the pseudo-second-order kinetic mode.
A greater pseudo-second-order rate constant of 0.6142 g·mg-1·h-1was achieved by ESIX, which was higher than that of 0.2632 g·mg-1·h-1by IX.












Follow WeChat


