Sunshine Factory, Co., Ltd. > Applications > ZrP for CatalystZrP for Catalyst
Conversion of biomass to chemicals over zirconium phosphate-based catalysts
Various glycerol conversion techniques have been developed and glycerol has become a potential renewable resource for the production of various high-value-added chemicals.
A representative chemocatalytic conversion of biomass to high-value-added products is summarized in Fig. 1.

Product Description
Zirconium phosphate (ZrP) is a family of transition-metal phosphate materials.in particular, have high thermal stabilities and water tolerances, and form sediments easily , and their medium to strong acidities make them compatible with polar media, including aqueous phases. These properties are advantageous in biomass conversions, which always involve a high temperature and aqueous media.
Advantage
α-ZrP has been most widely investigated and used in catalytic reactions. It is a typical layered compound with a well-defined structure and has both medium-strength acidic (-8.2 < H0 ≤ -3.0) and weak acidic sites (-3.0 < H0 ≤+6.8). The acid density is about 0.93 mmol/g, determined using the Hammett indicator method, and the surface area is ca.27.5 m2/g. Because of its particular acidic and porous properties, α-ZrP can act as both a catalyst support and an efficient catalyst directly
1. Mesoporous ZrP has useful properties such as a high surface area, narrow size distribution, specific particle morphology, and high acid strength. Large pores aid diffusion of large organic molecules, enhancing the catalytic ability.
2. The high catalytic activity can be attributed to a large surface area and the presence of a large number of acidic sites on the material surface. The catalysts show negligible loss of catalytic activity in stability tests, suggesting that the mesoporous ZrP framework has high chemical stability and the catalyst can be recycled.
3. The thermal stability of the acidic sites on the resulting metal phosphates enables their use under harsh conditions such as high temperature and in the presence of water-steam.
4. Furthermore, the acidic sites in the ZrP skeletal structure are flexible and adjustable. It should be noted that because of the synergistic effect of combining metal centers and acidic sites in metal-modified ZrP catalysts, they have a broad range of potential applications in a large number of specific reactions.
Product Structure
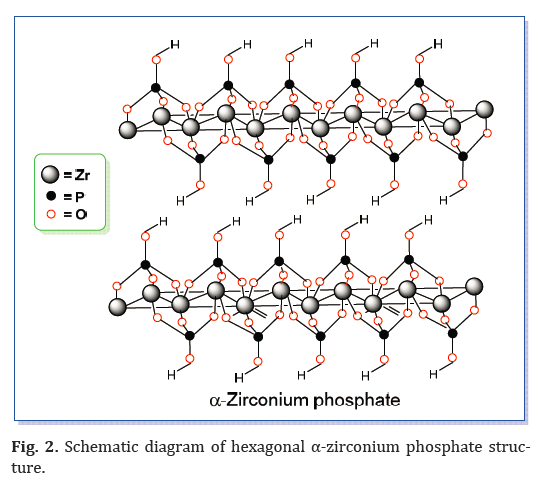
The ZrP layered structure consists of zirconium ions in a semi-planar arrangement, located slightly above and below the mean plane. Each Zr4+ ion is connected through the oxygen atoms of phosphate groups above and below (Fig. 2).
Catalytic applications of Zirconium Phosphate
we focus on their recent applications in catalytic transformations of biomass-derived platform molecules via reactions such as dehydration, hydrogenation, hydrogenolysis, and oxidation, and also clarify the role of the surface active sites of ZrP catalysts.
Fine chemicals via biomass dehydration
ZrP-derived catalysts are widely used in the preparation of fine chemicals via biomass dehydration because of their medium-strength acidic sites and the high thermal stability of the ZrP structure. The Brønsted and Lewis acidic sites can be adjusted for specific catalytic reactions.
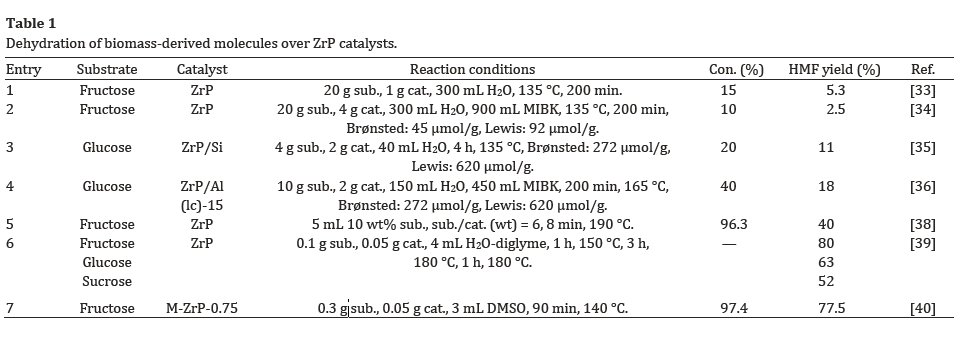
These results for catalytic dehydration over ZrP catalysts are summarized in Table 1.
A representative chemocatalytic conversion of biomass to high-value-added products is summarized in Fig. 1.

Product Description
Zirconium phosphate (ZrP) is a family of transition-metal phosphate materials.in particular, have high thermal stabilities and water tolerances, and form sediments easily , and their medium to strong acidities make them compatible with polar media, including aqueous phases. These properties are advantageous in biomass conversions, which always involve a high temperature and aqueous media.
Advantage
α-ZrP has been most widely investigated and used in catalytic reactions. It is a typical layered compound with a well-defined structure and has both medium-strength acidic (-8.2 < H0 ≤ -3.0) and weak acidic sites (-3.0 < H0 ≤+6.8). The acid density is about 0.93 mmol/g, determined using the Hammett indicator method, and the surface area is ca.27.5 m2/g. Because of its particular acidic and porous properties, α-ZrP can act as both a catalyst support and an efficient catalyst directly
1. Mesoporous ZrP has useful properties such as a high surface area, narrow size distribution, specific particle morphology, and high acid strength. Large pores aid diffusion of large organic molecules, enhancing the catalytic ability.
2. The high catalytic activity can be attributed to a large surface area and the presence of a large number of acidic sites on the material surface. The catalysts show negligible loss of catalytic activity in stability tests, suggesting that the mesoporous ZrP framework has high chemical stability and the catalyst can be recycled.
3. The thermal stability of the acidic sites on the resulting metal phosphates enables their use under harsh conditions such as high temperature and in the presence of water-steam.
4. Furthermore, the acidic sites in the ZrP skeletal structure are flexible and adjustable. It should be noted that because of the synergistic effect of combining metal centers and acidic sites in metal-modified ZrP catalysts, they have a broad range of potential applications in a large number of specific reactions.
Product Structure
The ZrP layered structure consists of zirconium ions in a semi-planar arrangement, located slightly above and below the mean plane. Each Zr4+ ion is connected through the oxygen atoms of phosphate groups above and below (Fig. 2).

These hydroxyl groups connected with phosphorus atoms are responsible for the Brønsted acidity and the zirconium atom centers provide the Lewis acidity. The Brønsted and Lewis acidities are controlled by changing the phosphorus and zirconium sources. α-ZrP has high cation-exchange capabilities of ca. 6.64 mmol H+/g respectively, because of their high densities of acidic sites and reactive surface hydroxyl groups. The interlayer distances in α-ZrP is 0.76 nm.
Catalytic applications of Zirconium Phosphate
we focus on their recent applications in catalytic transformations of biomass-derived platform molecules via reactions such as dehydration, hydrogenation, hydrogenolysis, and oxidation, and also clarify the role of the surface active sites of ZrP catalysts.
Fine chemicals via biomass dehydration
ZrP-derived catalysts are widely used in the preparation of fine chemicals via biomass dehydration because of their medium-strength acidic sites and the high thermal stability of the ZrP structure. The Brønsted and Lewis acidic sites can be adjusted for specific catalytic reactions.
These results for catalytic dehydration over ZrP catalysts are summarized in Table 1.


Follow WeChat


