Sunshine Factory, Co., Ltd. > Applications > ZrP for CatalystZrP for Catalyst
Transition Metal-Modified Zirconium Phosphate Electrocatalysts for the Oxygen Evolution Reaction
Zirconium phosphate (ZrP), an inorganic layered nanomaterial, is currently being investigated as a catalyst support for transition metal-based electrocatalysts for the oxygen evolution reaction (OER).
The use of the zirconium phosphate (ZrP) type of layered inorganic compounds, as a support for water oxidation catalysts, is examined.

Electrochemical Studies of Metal-Modified ZrP
One figure of merit conventionally used to describe OER catalyst performance is the overpotential necessary to reach a current density of 10 mA/cm 2.
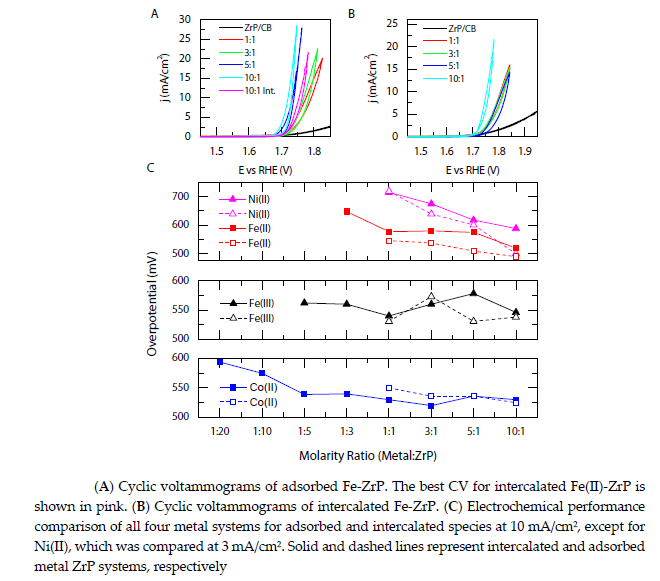
Figures A,B show cyclic voltammograms for the highest performance metal-modified ZrP system with either Fe(II) adsorbed or intercalated, while the other systems can be found in the below Figure.
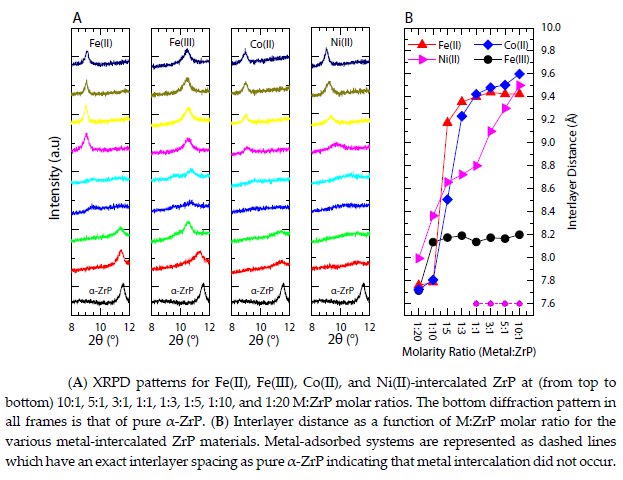
For reference, pure α-ZrP loaded onto carbon black shows only moderate activity for the OER, unable to achieve 10 mA/cm2 even at high oxidative potentials, which is comparable to other Zr-based systems.
Once metal cations are ion exchanged into and/or supported onto ZrP, an active OER catalyst is produced for both metalmodified ZrP systems at various M:ZrP ratios.
Adsorbed Fe(II)-ZrP shows a gradual increase in performance as the M:ZrP concentration increases and similar results are observed for the intercalated case.
As shown in Figure, higher metal content is achieved at higher M:ZrP molar ratios for both systems; therefore, it is likely that this gradual increase in activity can be attributed to higher atomic concentrations.
The adsorbed 10:1 Fe(II):ZrP molar ratio exhibits the highest OER performance of all the systems investigated herein,
requiring an overpotential of 490 mV at 10 mA/cm2 with a 0.55:1 Fe:Zr atomic ratio, as determined by XPS. For comparison, the metalintercalated equivalent system of 10:1 Fe(II): ZrP (with a higher Fe:Zr atomic ratio of 0.63:1) requires a higher overpotential of 524 mV, suggesting that the metal intercalated within ZrP may not be electrochemically accessible for water oxidation.

Electrochemical Studies of Metal-Modified ZrP
To study the electrocatalytic activity of metal-modified ZrP systems for the OER, cyclic voltammetry (CV) measurements were performed in a three-electrode rotating disk electrode (RDE) configuration with a 0.1 M KOH electrolyte. To make the working electrode, the metal-modified ZrP catalysts were mixed with carbon black in a water-based solution and deposited onto a glassy carbon disk.
One figure of merit conventionally used to describe OER catalyst performance is the overpotential necessary to reach a current density of 10 mA/cm 2.
Figures A,B show cyclic voltammograms for the highest performance metal-modified ZrP system with either Fe(II) adsorbed or intercalated, while the other systems can be found in the below Figure.

For reference, pure α-ZrP loaded onto carbon black shows only moderate activity for the OER, unable to achieve 10 mA/cm2 even at high oxidative potentials, which is comparable to other Zr-based systems.
Once metal cations are ion exchanged into and/or supported onto ZrP, an active OER catalyst is produced for both metalmodified ZrP systems at various M:ZrP ratios.
Adsorbed Fe(II)-ZrP shows a gradual increase in performance as the M:ZrP concentration increases and similar results are observed for the intercalated case.
As shown in Figure, higher metal content is achieved at higher M:ZrP molar ratios for both systems; therefore, it is likely that this gradual increase in activity can be attributed to higher atomic concentrations.
The adsorbed 10:1 Fe(II):ZrP molar ratio exhibits the highest OER performance of all the systems investigated herein,
requiring an overpotential of 490 mV at 10 mA/cm2 with a 0.55:1 Fe:Zr atomic ratio, as determined by XPS. For comparison, the metalintercalated equivalent system of 10:1 Fe(II): ZrP (with a higher Fe:Zr atomic ratio of 0.63:1) requires a higher overpotential of 524 mV, suggesting that the metal intercalated within ZrP may not be electrochemically accessible for water oxidation.
Figure C shows electrochemical data comparing the other metal-modified ZrP systems. All metal-modified systems are catalytically active, requiring between 0.5 and 0.7 V of overpotential to reach 10 mA/cm2, depending on the choice of the metal cation, the M:ZrP molar ratio used during synthesis, and whether the metal was intercalated into, or adsorbed onto, ZrP.
In general, lower overpotentials are observed for the higher M:ZrP molar ratios, ascribed to higher metal loadings.
For the Fe(II): ZrP and Ni(II): ZrP catalysts, the intercalated systems consistently require higher overpotentials versus their adsorbed counterparts, e.g, by 34 mV and 88 mV, respectively, at a M:ZrP ratio of 10:1. A less pronounced difference in activity for the two types of metal-modification was observed for the cases of Fe(III):ZrP and Co(II):ZrP.
Given that, in general, OER activities for the metal-adsorbed ZrP catalysts are greater than or equal to those of their metal-intercalated counterparts at the same loading, it is likely that the OER is dominated by catalysis on the outer surface of the ZrP supported metal-based systems rather than within the layers, which may be limited by mass transport.
Given that, in general, OER activities for the metal-adsorbed ZrP catalysts are greater than or equal to those of their metal-intercalated counterparts at the same loading, it is likely that the OER is dominated by catalysis on the outer surface of the ZrP supported metal-based systems rather than within the layers, which may be limited by mass transport.

Follow WeChat


