Sunshine Factory, Co., Ltd. > Applications > ZrP for Fuel Cell, Lithium BatZrP for Fuel Cell, Lithium Bat
Zirconium phosphate used in lithium ion batteries as high voltage cathode material
Abstract
To solve the major problem of interfacial side reactions between LiMn 1.5 Ni0.5O4 (LMNO) and liquid electrolyte at high voltages, zirconium phosphate (ZrP) wrapped cathode material LMNO@ZrP, with different aspect of ZrP in 2 wt% and 4 wt%, have been prepared.
The effects of ZrP coating layer have been investigated by comparison of the properties of the ZrP coated LMNO (LMNO@ZrP) and pristine LMNO electrodes by several electrochemical characterizations.
The electrochemical impedance spectroscopy results show that LMNO@ZrP-3M-2 electrode exhibits an improved electrochemical property of suppressing the impedance increase.
In the cell life test, LMNO@ZrP-3M-2 electrode shows a high capacity retention of 94.6% after 200 cycles at 55 ℃.
Experimental

Results and discussion
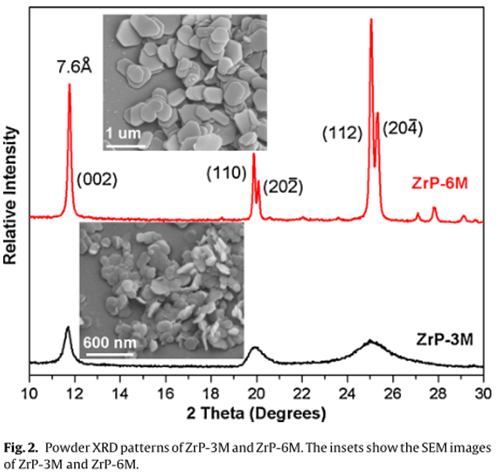
Physicochemical characterization of LMNO@ZrP


Electrochemical properties

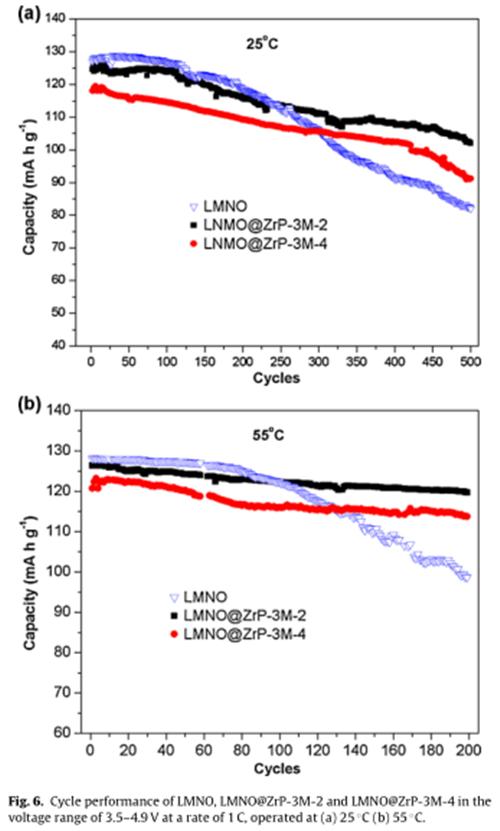
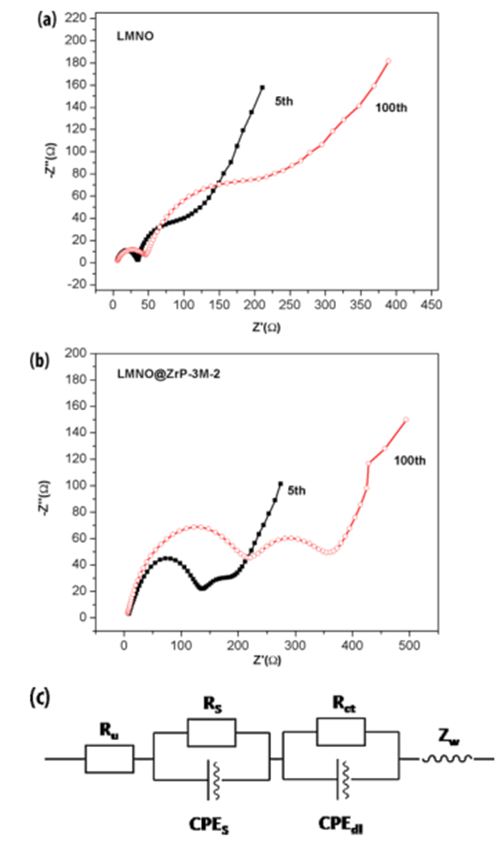
Fig.7. Nyquist plots obtained by electrochemical impedance for lithium ion. batteries with cathode electrodes of (a) pristine LMNO and (b) LMNO@ZrP-3M-2 after 5 cycles and 100 cycles; (c) the corresponding equivalent circuit.
The impedance values were summarized ,as below:
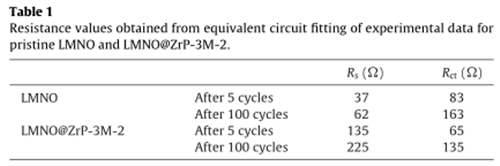
For pristine and ZrP-3M coated LMNO, the results reveal that the Rs and R ct values increased after cycling. It can be seen that the introduction of coating layers can decrease the lithium-ion mobility through the surface layer of cathode materials, and in turn increase the surface resistance Rs .
On the other hand, significant increases of Rct values are mainly due to the side reactions between cathode materials and non-aqueous electrolytes during cycling.
Conclusion
Nevertheless,the cells with the non-coated LMNO exhibited a dramatic capacity decrease from the 80th cycle and the capacity retention was only 77.1% after 200 cycles.
The physical protection layer of ZrP can effectively prevent or suppress the chemical reactions between cathode materials and non-aqueous electrolytes, reduce charge transfer impedance, and enhance cycling stability.
Direct surface modification of LMNO with ZrP nanosheets provides a convenient and economical approach to improve the comprehensive properties of cathode material used for lithium-ion battery applications.
To solve the major problem of interfacial side reactions between LiMn 1.5 Ni0.5O4 (LMNO) and liquid electrolyte at high voltages, zirconium phosphate (ZrP) wrapped cathode material LMNO@ZrP, with different aspect of ZrP in 2 wt% and 4 wt%, have been prepared.
The effects of ZrP coating layer have been investigated by comparison of the properties of the ZrP coated LMNO (LMNO@ZrP) and pristine LMNO electrodes by several electrochemical characterizations.
The electrochemical impedance spectroscopy results show that LMNO@ZrP-3M-2 electrode exhibits an improved electrochemical property of suppressing the impedance increase.
In the cell life test, LMNO@ZrP-3M-2 electrode shows a high capacity retention of 94.6% after 200 cycles at 55 ℃.
Experimental

Results and discussion
Physicochemical characterization of LMNO@ZrP


Fig. 4 shows the SEM and TEM images of the LMNO and LMNO@ZrP powders. Pristine LMNO shows smooth planes and well-defined edges.

SEM photographs of (a) LMNO@ZrP-3M-2, (b) LMNO@ZrP-6M-2, (c) LMNO@ZrP-3M-4, (d) LMNO@ZrP-6M-4 and (e) pristine LMNO.(f) A TEM photographs of LMNO@ZrP-3M-2.
Electrochemical properties


Fig.7. Nyquist plots obtained by electrochemical impedance for lithium ion. batteries with cathode electrodes of (a) pristine LMNO and (b) LMNO@ZrP-3M-2 after 5 cycles and 100 cycles; (c) the corresponding equivalent circuit.

The impedance values were summarized ,as below:

For pristine and ZrP-3M coated LMNO, the results reveal that the Rs and R ct values increased after cycling. It can be seen that the introduction of coating layers can decrease the lithium-ion mobility through the surface layer of cathode materials, and in turn increase the surface resistance Rs .
On the other hand, significant increases of Rct values are mainly due to the side reactions between cathode materials and non-aqueous electrolytes during cycling.
Conclusion
The morphology observation shows that ZrP with appropriate lateral dimension can well wrap on LMNO particles and then modify the cathode material.
The experiment results indicate that the surface modified cathode materials exhibit better performance when compared with pristine LMNO.
Although the adding of electrochemical inert ZrP-3M slightly decreased the discharge capacities, in the cell life test, LMNO@ZrP-3M-2 electrode exhibited a capacity retention of 94.6% after 200 cycles at 55℃.
The experiment results indicate that the surface modified cathode materials exhibit better performance when compared with pristine LMNO.
Although the adding of electrochemical inert ZrP-3M slightly decreased the discharge capacities, in the cell life test, LMNO@ZrP-3M-2 electrode exhibited a capacity retention of 94.6% after 200 cycles at 55℃.
Nevertheless,the cells with the non-coated LMNO exhibited a dramatic capacity decrease from the 80th cycle and the capacity retention was only 77.1% after 200 cycles.
The physical protection layer of ZrP can effectively prevent or suppress the chemical reactions between cathode materials and non-aqueous electrolytes, reduce charge transfer impedance, and enhance cycling stability.
Direct surface modification of LMNO with ZrP nanosheets provides a convenient and economical approach to improve the comprehensive properties of cathode material used for lithium-ion battery applications.
Contents from School of Chemistry and Environment, South China Normal University, Science Building No. 1, Guangzhou 510006, China

Follow WeChat


