Sunshine Factory, Co., Ltd. > Applications > ZrP for PolymerZrP for Polymer
Preparation and Characterisation of a Novel Fire Retardant PET/α-Zirconium Phosphate Nanocomposite
Abstract:
The preparation of a novel fire retardant nanocomposite of poly (ethylene terephthalate) (PET)/α-zirconium phosphate (α-ZrP) by situ polymerization was investigated.
The novel fire retarded PET nanocomposite, PET-co-DDP/α-ZrP nanocomposite, was synthesized by the direct condensation of terephthalic acid, ethylene glycol, 9,10-dihydro-10[2,3-di(hydroxycarbonyl)propyl]-10-phosphaphenanthrene-10-oxide and nano α-ZrP. The morphology, thermal stability and burning behaviour of the nanocomposite with 1 wt-% α-ZrP loadings were investigated.
The novel fire retarded PET nanocomposite, PET-co-DDP/α-ZrP nanocomposite, was synthesized by the direct condensation of terephthalic acid, ethylene glycol, 9,10-dihydro-10[2,3-di(hydroxycarbonyl)propyl]-10-phosphaphenanthrene-10-oxide and nano α-ZrP. The morphology, thermal stability and burning behaviour of the nanocomposite with 1 wt-% α-ZrP loadings were investigated.
The extent of dispersion of the nanofillers was quantified by X-ray diffraction, scanning electron microscopy and transmission electron microscopy.
Significant improvements in fire retardant performance were observed for the nanocomposite from limiting oxygen index (increased from 21.2 to 32.6), UL-94 (achieving V-0), and cone calorimetry (reducing both the heat release rate and the total heat released, without reducing the time to ignition).
Preparation of PET-co-DDP/α-ZrP nanocomposites
PET-co-DDP/α-ZrP nanocomposite was prepared from TPA, EG, DDP and modified α-ZrP by direct condensation polymerisation.
The synthetic route was as follows: initially, the modified α-ZrP (1wt %) was added to EG (200 ml) and stirred in an ultrasonic bath for 0.5 hour as pretreatment. After this, TPA, EG, DDP and the pretreated α-ZrP solutions were introduced into a reactor equipped with a nitrogen inlet, a condenser and a mechanical stirrer.
The reactor was heated to 240℃under high pressure (0.4-0.5MPa) and maintained for 3.5 h. After this, the pressure of the
The synthetic route was as follows: initially, the modified α-ZrP (1wt %) was added to EG (200 ml) and stirred in an ultrasonic bath for 0.5 hour as pretreatment. After this, TPA, EG, DDP and the pretreated α-ZrP solutions were introduced into a reactor equipped with a nitrogen inlet, a condenser and a mechanical stirrer.
The reactor was heated to 240℃under high pressure (0.4-0.5MPa) and maintained for 3.5 h. After this, the pressure of the
reactor was reduced to less than 100 Pa and maintained for 2 h. A comparison material, PET-co-DDP with 1wt% phosphorous was prepared from TPA, EG and DDP according to the procedure reported by Chang et al.
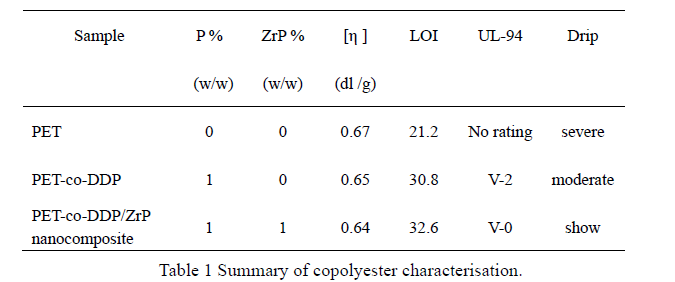
The intrinsic viscosities (η) of the copolyesters was determined with an Ubbelohde viscometer at 30oC in phenol/ 1,1,2,2 - tetrachloroethane (60/40, w/w) solution; the results are given in Table 1.
The intrinsic viscosities (η) of the copolyesters was determined with an Ubbelohde viscometer at 30oC in phenol/ 1,1,2,2 - tetrachloroethane (60/40, w/w) solution; the results are given in Table 1.
Results and discussion
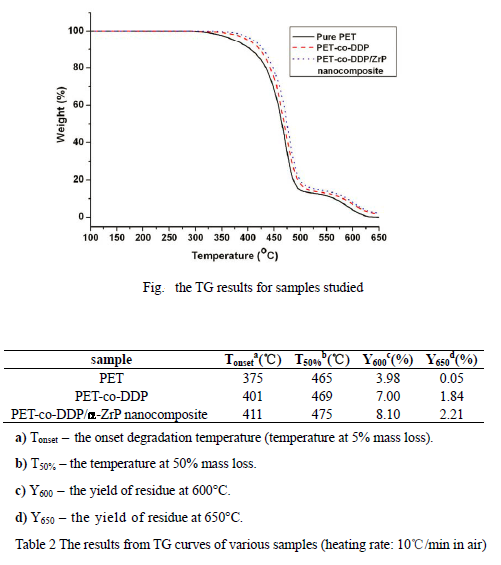
Thermal stability
Burning behaviour
1. Small flame tests
Limiting oxygen index (LOI) and Bunsen burner tests such as the UL-94 are widely used to evaluate fire retardant formulations.
The LOI and UL-94 classification of each sample was determined, as shown in table 1. It can be observed that the LOI value of PET increased from 21.2 to 30.8 when 1 wt% P was present in the copolymer, and the UL-94 reached V-2, due to flaming drips.
The LOI and UL-94 classification of each sample was determined, as shown in table 1. It can be observed that the LOI value of PET increased from 21.2 to 30.8 when 1 wt% P was present in the copolymer, and the UL-94 reached V-2, due to flaming drips.
However, the PET-co-DDP/α-ZrP nanocomposite gave an LOI of 32.6, and a UL-94 rating of V-0, with a total nanoparticle content of only 1%. This may be due to an increase in the melt viscosity near the ignition temperature, reducing the dripping
tendency as a result of the presence of a well-dispersed nanofiller.
Meanwhile, the metal in the nanoparticle may have catalyzed the carbonization of the polymer forming a more effective char layer, reducing the transfer of heat and fuel.
2. Cone calorimetry
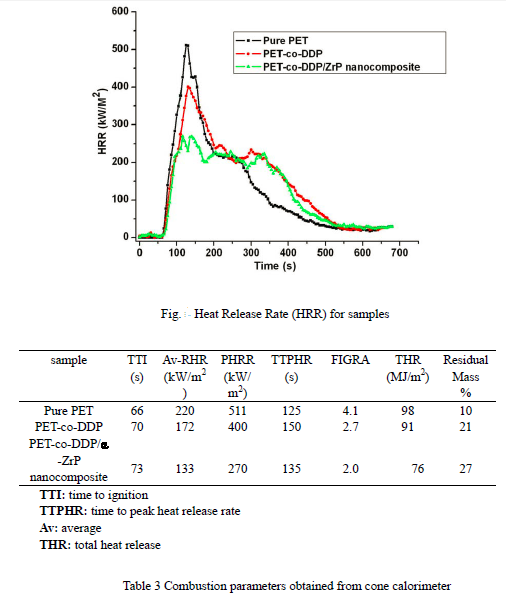
The cone calorimeter is a bench-scale fire test apparatus and provides a wealth of information on combustion behaviour. Some cone calorimeter results have been found to correlate well with those obtained from large scale fire tests, so that it can be used to predict the behaviour of materials in a real fire. It is a useful tool for the evaluation of fire retardant materials and quantifies fire parameters such as time to ignition (TTI), heat release rate (HRR), and total heat release (THR). The Figure shows curves of the heat release rate (HRR) against time of pure PET, PET-co-DDP and PET-co-DDP/ZrP nanocomposite. Pure PET burns rapidly after ignition and a sharp HRR peak appears with a peak heat release rate (PHRR) of 511kW/m2 while
Meanwhile, the metal in the nanoparticle may have catalyzed the carbonization of the polymer forming a more effective char layer, reducing the transfer of heat and fuel.
2. Cone calorimetry
The cone calorimeter is a bench-scale fire test apparatus and provides a wealth of information on combustion behaviour. Some cone calorimeter results have been found to correlate well with those obtained from large scale fire tests, so that it can be used to predict the behaviour of materials in a real fire. It is a useful tool for the evaluation of fire retardant materials and quantifies fire parameters such as time to ignition (TTI), heat release rate (HRR), and total heat release (THR). The Figure shows curves of the heat release rate (HRR) against time of pure PET, PET-co-DDP and PET-co-DDP/ZrP nanocomposite. Pure PET burns rapidly after ignition and a sharp HRR peak appears with a peak heat release rate (PHRR) of 511kW/m2 while
the PHRR of PET-co-DDP and PET-co-DDP/α-ZrP nanocomposite are 400 and 270kW/m2, respectively. It should be noted that the PHRR of nanocomposite is only 52.8% of that of pure PET. The detailed data is shown in table 3.

The samples studied also showed considerable differences in the Total Heat Release (THR/Time) curves presented in below figure, most importantly showing that both DDP and the α-ZrP nanocomposite reduced the total amount of fuel available for
combustion.
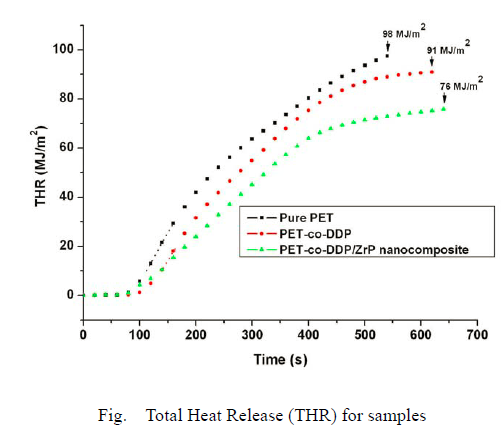
At the end of burning, pure PET has released a total heat of 98 MJ/m2, the PET-co-DDP has released is 91 MJ/m2, whereas only 76 MJ/m2 has been released by the PET-co-DDP/α-ZrP nanocomposite. This latter significant difference results from the addition of 1% nanoparticles to the polymer, and indicates that part of the copolymer has not completely combusted, probably undergoing a partial carbonization process. This is in accord with the reduced mass loss, presented in below figure.
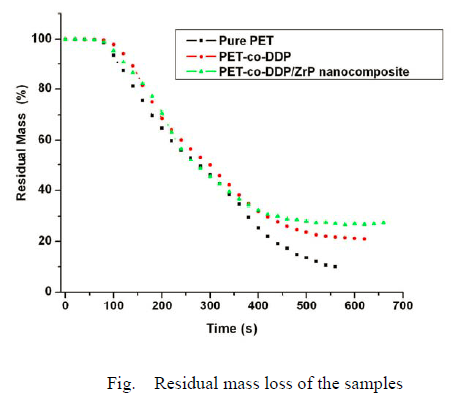
It shows mass loss as a function of combustion time for pure PET, PE-co-DDP and PET-co-DDP/α-ZrP nanocomposite. The PET-co-DDP/α-ZrP nanocomposite shows a significantly lower mass loss and the higher char yields correspond to the lower THR. This indicates a condensed phase mechanism for the fire retardancy of DDP and α-ZrP. It is particularly interesting to note that this enhanced char performance was less evident in the TG experiments, but was clearly visible under the more severe conditions of the cone calorimeter at 50 kW/m2.
One method for summarising the flammability of the fuel from the HRR curve generated by cone calorimetry is the slope of the FIGRA (fire growth rate) line, extending from the origin to the earliest, highest peak, providing an estimation of both the spread rate and the size of a fire, and used to estimate the contribution to fire growth of materials. FIGRA for the samples is shown in table 3. A lower value indicates a slower fire growth rate and implies lower flammability. FIGRA of PET-co-DDP/α-ZrP nanocomposite is 2, better than PET at 4.1 or PET-co-DDP at 2.7.
Conclusions
The morphology of the nanocomposite has been investigated by XRD, SEM and TEM. The effect of small amounts of nanoparticle (α-ZrP) on the thermal stability and burning behaviour is apparent; it shows inhibition of thermal decomposition and higher char yields. From 15 cone calorimetry, the nanocomposite shows a very significant decrease in HRR, PHRR, THR and FIGRA compared to both the pure PET and a fire retarded PET without nanofiller.
The fire retardant mechanism observed in the cone calorimeter is char formation, slowing the initial decomposition, and increasing the final char yield.
The fire retardant mechanism observed in the cone calorimeter is char formation, slowing the initial decomposition, and increasing the final char yield.
In contrast to many polymer nanocomposites, which shows a shorter time to ignition and a lower heat release rate and peak of HRR, in this case formation of a nanocomposite increases the time to ignition, while simultaneously reducing both the PHRR and the THR. This may be related to the formation of the nanostructure which can reduce the transfer of heat and fuel in the burning process, at the same time, the metal in the nanoparticle might catalyse the reaction leading to graphitization, but this
has not been demonstrated in the evidence presented here. Further work will be needed to study and understand the fire retardant mechanism and obtain more direct evidence.

Follow WeChat


