Sunshine Factory, Co., Ltd. > Applications > ZrP for PolymerZrP for Polymer
The most efficient flame-retardant additives of high impact polystyrene.
Abstract
High-impact polystyrene (HIPS)/organophilic alpha-zirconium phosphate (organophilic α-ZrP, OZrP) nanocomposites were prepared by melt blending method.
High-impact polystyrene (HIPS)/organophilic alpha-zirconium phosphate (organophilic α-ZrP, OZrP) nanocomposites were prepared by melt blending method.
The XRD and high-resolution transmission electronic microscopy results showed that an intercalated/exfoliated structure of nanocomposites was formed.
The thermal degradation behaviors indicated that the HIPS/OZrP nano-composites displayed similar thermo-oxidative stability with that of HIPS, and α-ZrP could promote the formation of char residue of the nanocomposites.
The combustion behaviors revealed that the addition of α-ZrP could reduce the value of peak heat release rate (HRR) and mass loss rate (MLR) of the nanocomposites.
The peak HRR value of HIPS/OZrP nanocomposites with 3 wt% OZrP was 17 % lower than that of pure HIPS.
The trends of MLR were the same as those of the HRR, which indicated that the mechanism of the observed reduction both in HRR and MLR by addition of the layered phosphate depended mainly on the condensed phase flame-retardant process instead of the gas phase flame-retardant process.
It could be observed that HIPS left no residues after burning, whereas the HIPS/OZrP nanocomposites left the consistent and compact char.
Experimental
The pristine α-ZrP is hydrophilic and the HIPS matrix is hydrophobic.
To obtain a thermodynamically homogeneous mixture of the pristine α-ZrP with polymer matrix on the molecular level, α-ZrP was organically treated with C16 before melt intercalation by polymer.
The OZrP powder and HIPS pellets were dried under vacuum at 80 °C for overnight before using.
A Brabender mixer of 50 g capacity was used for the preparation of all samples at 180 °C for 10–15 min at 90 rpm, followed by hot-pressing the as-prepared HIPS composites under 10 MPa for 10 min at 180 °C into sheets of 3 mm thick, which were subsequently cut into suitable size for various analyses.
During the blending procedure, the OZrP powder was added directly to hot and melted HIPS.
Table 1 shows the mixing weight ratio of the samples.
Results and discussion
Flammability properties
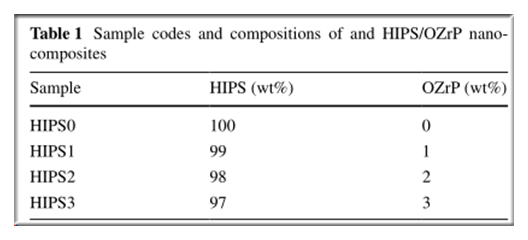
1.The HRR peak values
However,when the OZrP loading amount is 1 wt% (HIPS1), the ignition time decreases from 64 s (HIPS0) to 47 s.
The initial HRR for the HIPS1 is higher in the first several seconds following ignition (about 47–85 s from the onset), probably because of the thermal decomposition of its organic emulsifier resulting in the formation of volatile combustibles.
When the OZrP loading amount is 3 wt% (HIPS3),the ignition time increases slightly from 64 s (HIPS0) to 65 s.
The reason can be postulated that increased phosphate can quickly promote the formation of charred layer in the condensed phase on the initial stage of the polymer degradation process, which then delays the onset of combustion and reduces the combustion speed.
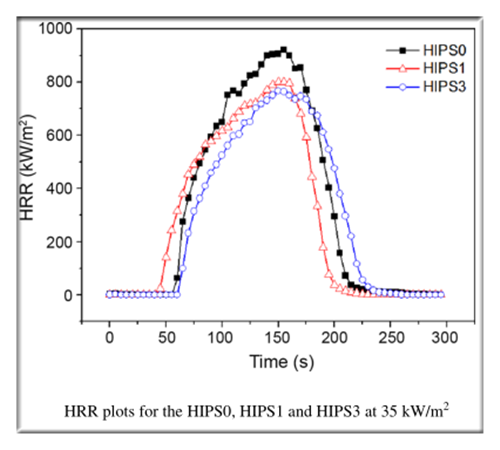
2.The MLR
The above figure shows that the MLR decreases in the order of HIPS3 > HIPS1 > HIPS0, this trend is the same as that of the HRR in the CCT .
These similarities indicate that the mechanism of the observed reduction both in HRR and MLR by addition of the layered phosphate depends mainly on the condensed phase flame-retardant process instead of the gas phase flame-retardant process.
Namely, it reveals that the exfoliated phosphate nanometer sheets dispersed in polymer matrix can effectively reduce the mass loss rate as well as prevent the heat release rate by increasing the char formation.
The char formed layers may act as insulator and a mass transport barrier slowing the escape of the volatile products generated as the HIPS decomposes.
To obtain a thermodynamically homogeneous mixture of the pristine α-ZrP with polymer matrix on the molecular level, α-ZrP was organically treated with C16 before melt intercalation by polymer.
The OZrP powder and HIPS pellets were dried under vacuum at 80 °C for overnight before using.
A Brabender mixer of 50 g capacity was used for the preparation of all samples at 180 °C for 10–15 min at 90 rpm, followed by hot-pressing the as-prepared HIPS composites under 10 MPa for 10 min at 180 °C into sheets of 3 mm thick, which were subsequently cut into suitable size for various analyses.
During the blending procedure, the OZrP powder was added directly to hot and melted HIPS.
Table 1 shows the mixing weight ratio of the samples.

Results and discussion
Flammability properties
1.The HRR peak values
The HRR peak values of HIPS/OZrP nanocomposites corresponding to the OZrP content of 1 wt% (HIPS1) and 3 wt% (HIPS3) are 13 and 17 % lower than those of pure HIPS (HIPS0), respectively.
However,when the OZrP loading amount is 1 wt% (HIPS1), the ignition time decreases from 64 s (HIPS0) to 47 s.
The initial HRR for the HIPS1 is higher in the first several seconds following ignition (about 47–85 s from the onset), probably because of the thermal decomposition of its organic emulsifier resulting in the formation of volatile combustibles.
When the OZrP loading amount is 3 wt% (HIPS3),the ignition time increases slightly from 64 s (HIPS0) to 65 s.
The reason can be postulated that increased phosphate can quickly promote the formation of charred layer in the condensed phase on the initial stage of the polymer degradation process, which then delays the onset of combustion and reduces the combustion speed.

2.The MLR

The above figure shows that the MLR decreases in the order of HIPS3 > HIPS1 > HIPS0, this trend is the same as that of the HRR in the CCT .
These similarities indicate that the mechanism of the observed reduction both in HRR and MLR by addition of the layered phosphate depends mainly on the condensed phase flame-retardant process instead of the gas phase flame-retardant process.
Namely, it reveals that the exfoliated phosphate nanometer sheets dispersed in polymer matrix can effectively reduce the mass loss rate as well as prevent the heat release rate by increasing the char formation.
The char formed layers may act as insulator and a mass transport barrier slowing the escape of the volatile products generated as the HIPS decomposes.
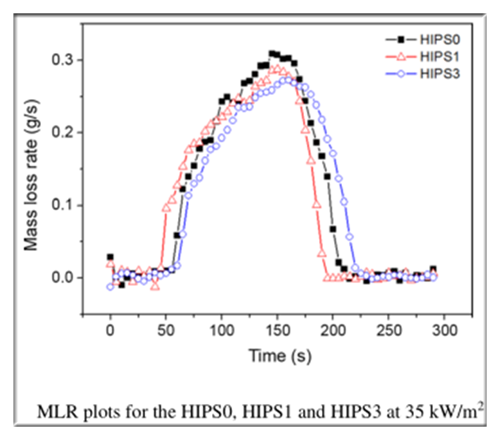
The photographs of the char residue samples collected after the CCT
It can be observed that pure HIPS (HIPS0) leaves no residues (Fig.a), whereas the residues are thin and loose charred layers from the HIPS1 sample (b) and consistent and compact charred layers from the HIPS3 sample ( c).
This also explains why the HIPS3 sample with phosphate has excellent flame retardant properties, because the compact charred layers can efficiently protect the underlying polymeric materials to be further burned.
This also explains why the HIPS3 sample with phosphate has excellent flame retardant properties, because the compact charred layers can efficiently protect the underlying polymeric materials to be further burned.
Catalytic combustion process and recommended reaction mechanism
HIPS layer phosphate nanocomposites have demonstrated improvement of flammability properties, namely the drop in heat release rate (HRR) peak and the mass loss rate, for mation of high degree of graphitization protective char and good gas barrier properties during combustion.
At present,several aspects are proposed to explain the catalytic combustion process.
1.First, HIPS chains can be introduced into the exfoliated OZrP gallery to form polymer/OZrP nanocomposites.OZrP, a solid acid, can thermally loose H+, which will attack molecular chains of HIPS to form cationic active sites, and results in catalytic degradation of HIPS and yields macroradicals.Many Lewis or Brӧnsted acid sites in the OZrP layer can further catch those macroradicals to recombine and lead to intermolecular crosslinking.The monomers such as butadiene and styrene, dimers and trimers coming from the degradation product of HIPS chain scission are crosslinked and aromatized to form graphite.
2.Simultaneously, many research works have shown that zirconium (Zr), as an element, can promote the graphitization of carbon and adding some amounts of Zr in carbon can assist higher degree of graphitization at a certain heat treatment temperature. When introduced into carbon substrate (such as HIPS), Zr reacts readily with carbon generated from the degradation of HIPS to form ZrC, the process may involve a dynamic thermodynamic equilibrium between ZrC and Zr, in which a conversion from carbon to graphite occurs.Part of the driving force may come from the free energy of the transformation from carbon to the stable graphite phase.
3.At last, the “labyrinth” effect, which is derived from the nano-dispersed phosphate layers in the polymer matrix and stable higher degree of graphitization char, can reduce the diffusion of both volatile thermo-oxidation products to the gas phase and oxygen from the gas to polymer.
As a result, the HIPS/OZrP nanocomposites showed a decrease of HRR and MLR, that is, an improvement of the flame retardancy.
(Artilce from Iran Polym J (2015) 24:1069–1075 DOI 10.1007/s13726-015-0394-4)

Follow WeChat


